In physical engineering and chemical science, there are mainly two types of chemical bonds. These are Ionic Bonds and Covalent bonds. In this exclusive article, I am going to explain the definition of Ionic Bond.
- Magnesium And Calcium Valence Electrons
- Valence Electrons Calculator
- Calcium Valence Electrons Count
- Calcium Valence Electrons
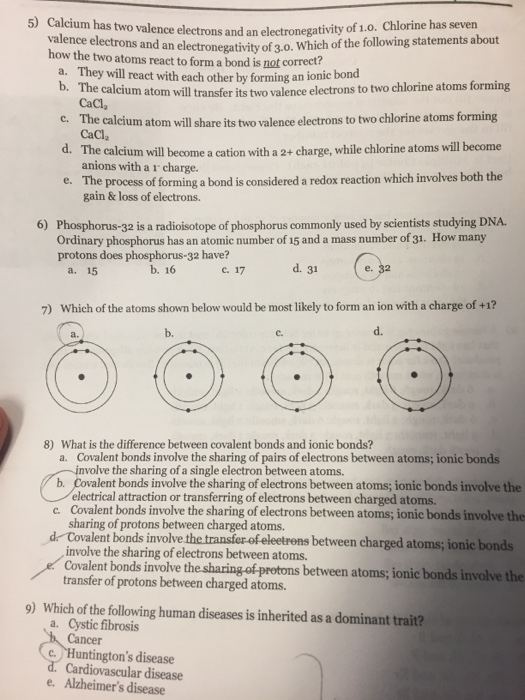
- Calcium Valence Electrons Gain Or Lose
Magnesium And Calcium Valence Electrons
However, I will still clear some air regarding the basic difference between covalent and ionic bonds. See, a covalent bond is sharing of electrons between two atoms. On the other hand, an ionic bond is a permanent transfer of valence electrons between two atoms.
Just to let you know, apart from ionic and covalent bonds, there are two more types of chemical bonds. These are hydrogen bonds and polar bonds.
Valence Electrons Calculator
What is Ionic Bond?
- There are 7 valence electrons because the highest energy level, 3, has 7 total electrons (5 plus 2 is 7). For calcium, which has an atomic number of 20 and therefore 20 electrons, find calcium on.
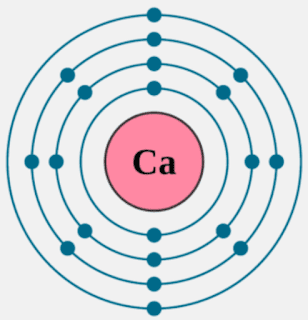
- Calcium is the third element in the second column of the periodic table. It is classified as an alkaline earth metal. Calcium atoms have 20 electrons and 20 protons. There are 2 valence electrons in the outer shell. Calcium is an important element for life on Earth and is the fifth most abundant element in the Earth's crust.
By definition, an Ionic bond is a type of chemical bond that occurs due to the permanent transfer of one or more electrons from one atom to another.
When adding calcium and oxygen together they for calcium oxide or cao and calciums 2 valence electrons join with oxygens 6 to fill the lewis structure. The outer electron of the sodium atom 281 is transferred to the outer shell of the chlorine atom 287 giving it a complete octet shell of outer electrons just like a noble gas 288. We know that the atomic number of calcium is 20.So calcium has 20 protons and 20 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Step-3: Now write the electron configuration of calcium. Ca (20)=1s²2s²2p⁶3s²3p⁶4s². Number of valence electrons in Calcium. August 27, 2014, charm, Leave a comment. How many valence electrons are there in Calcium? Do you like chemistry? Keep in mind the fact that Calcium can be found in the 4th energy level of the periodic table, and in the 2nd group (column).
The atom that permanently loses electrons becomes a cation i.e positively charged ion. On the other hand, the atom that gains electrons becomes an anion i.e negatively charged ion. That’s why an ionic bond is also known electrovalent bond.
Editor’s Choice: Covalent Bond – Definition, Types, Properties & Examples
To summarise, by definition, an ionic bond or simply electrovalent bond is a type of chemical bond that forms due to the electrostatic attraction between the positively charges cation and negatively charged anion.
Role of Electronegativity in Ionic Bond
For an ionic bond to form, there has to be a very large difference in the value of electronegativity between the participating atoms. As a result, the formation of ions takes place in ionic bonding.
And, for that to happen, ionic bonding can only form between metals and non-metals. To summarise, one can also say that two metals can never form an ionic bond. The same goes with non-metals too.

To put it differently, if we take two metals or non-metals, and try to form an ionic bond, it won’t form. WHY? Because there won’t be enough difference in the value of electronegativity of the participating atoms to form an ionic bond.
Properties of Ionic Bond
There are so many properties of ionic bonds. Some of them are listed below:
- By definition, Ionic bond is non-directional in nature.
- Ionic compounds are soluble in water and polar solvents.
- Ionic compounds are insoluble in non-polar solvents.
- They have the highest melting and boiling points.
- They show good electrical and thermal conductivity.
- Ionic bonds or electrovalent bonds are the strongest among all types of bonds.
- They form due to the permanent transfer of electrons, etc.
Examples of Ionic Bond
If you think you can’t relate to Ionic bond examples in everyday life. Well, here is your chance to think again!
Sodium Chloride (NaCl)
A typical example of an ionic bond is the Sodium Chloride molecule. A molecule of Sodium Chloride (NaCL) consists of one Sodium and one chlorine atom.
Sodium has one valence electron in its outermost shell. On the other hand, Chlorine has seven valence electrons in its outermost shell.
Therefore, when one atom of sodium and one atom of Chlorine combines to form an ionic bond, in order to complete its Octet, Sodium will donate its lone electron. Hence becomes sodium ions (Na+).
On the other hand, just because chlorine is more electronegative than sodium, it will accept the donated electron. Hence becomes chloride ions (Cl–). By this, two atoms combine to form an ionic bond-based compound i.e Sodium Chloride (NaCL).
Potassium Oxide (K2O)
The next example of an ionic bond is the Potassium Oxide molecule. A molecule of Potassium Oxide (K2O) consists of two potassium and one oxygen atom. Potassium has one valence electron in its outermost shell.
On the other hand, oxygen has six valence electrons in its outermost shell. Therefore, when two atoms of Potassium and one atom of oxygen combine to form an ionic bond, in order to complete its Octet, both of the Potassium atoms will donate their lone electron.
Hence becomes a Potassium ion (K+). On the other hand, just because Oxygen is more electronegative than Potassium, it will accept both of the donated electrons. Hence becomes oxide ion (O-).
By this, two atoms of potassium and one atom of oxygen combine to form an ionic bond-based compound i.e Potassium Oxide (K2O). Not to mention, just because there are two potassium atoms, the molecule of potassium oxide has two ionic bonds.
Calcium Chloride (CaCL2)
The last one in my list of examples of an ionic bond or electrovalent bond is the Calcium Chloride molecule. A molecule of Calcium chloride (CaCL2) consists of one calcium and two chlorine atoms. Calcium has two valence electrons in its outermost shell.
Similarly, chlorine has seven. Therefore, when one atom of calcium and two atoms of chlorine combine to form an ionic bond, in order to complete its Octet, the calcium atom will donate its two electrons. Hence becomes a calcium ion (Ca+).
On the other hand, just because chlorine is more electronegative than Calcium, both of the chlorine atoms will accept one each of the donated electrons. Hence becomes a chloride ion (Cl–).
By this, one atom of calcium and two atoms of chlorine combine to form an ionic bond-based molecule i.e Calcium Chloride (CaCL2). Not to mention, just because there are two chlorine atoms, the molecule of calcium chloride has two ionic bonds.
Uses of Ionic Compounds
Compounds that form due to ionic bonding is known as Ionic Compounds. Again, If you think you can’t relate to the uses of ionic compounds in everyday life. Well, here is your chance to think again!
- Sodium chloride as table salt
- De-icing of roads after snowfall
- As a preservative in cold storage
- Water fluoridation, etc.
At last, that’s it for this post. If you like this article, share it if you like, like it if you share it. You can also find us on Mix,Twitter,Pinterest, and Facebook.
Click to see full answer
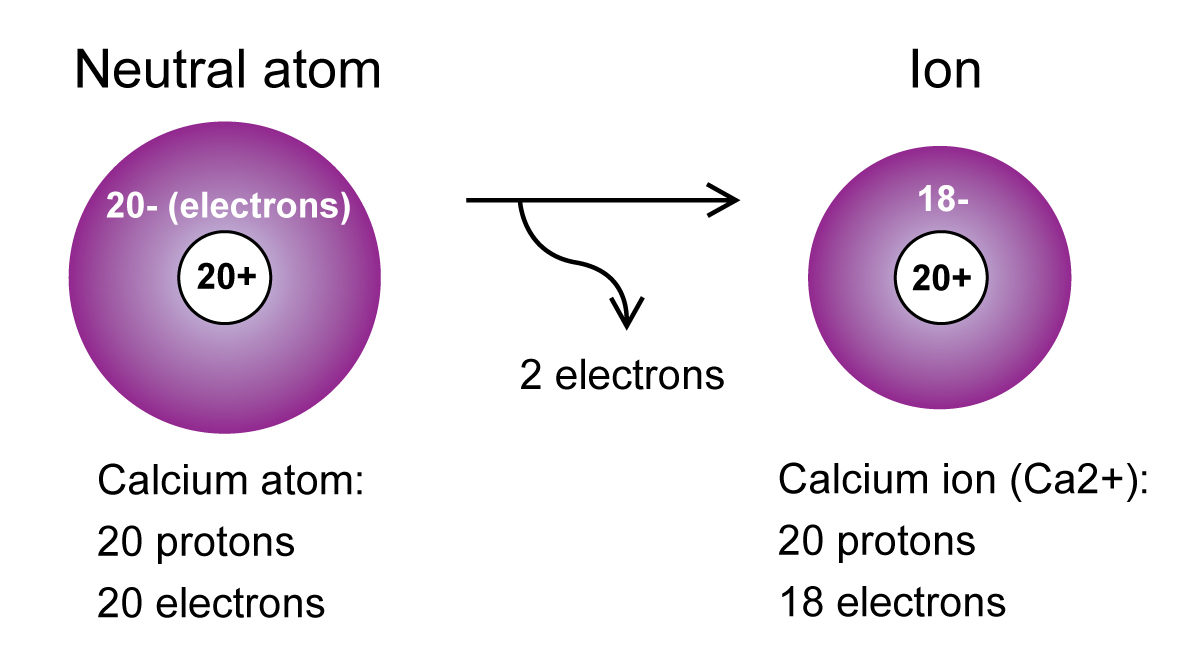
Besides, is calcium more likely to gain or lose electrons?
Some atoms gain or lose more than 1 electron. Calcium loses 2 electrons when it becomes an ion. Since calcium lost two electrons, it has 20 protons, but only 18 electrons. This makes calcium a positive ion with a charge of 2+.
Secondly, does chlorine gain or lose electrons? Chlorine (Cl) in its lowest energy state (called the ground state) has seven electrons in its outer shell. Again, it is more energy-efficient for chlorine to gain one electron than to lose seven. In the formation of an ionic compound, metals lose electrons and nonmetals gain electrons to achieve an octet.
Additionally, does beryllium lose or gain electrons?
The number of electrons an element will gain or lose depends upon its group number in the periodic table. Thus, Beryllium wants to lose two electrons. When it does that, Beryllium will have a positive charge of two, and it will be stated as B-e two plus.
Calcium Valence Electrons Count
Why elements lose or gain electrons?
Calcium Valence Electrons
Calcium Valence Electrons Gain Or Lose
Ions are formed when atoms lose or gain electrons in order to fulfill the octet rule and have full outer valence electron shells. When they lose electrons, they become positively charged and are named cations. When they gain electrons, they are negatively charged and are named anions.
