What is the valence electron configuration for calcium?
1 Answer

$begingroup$ What you provided is the ground-state configuration for calcium. In theory every atom 'has' d-electrons, just not in the ground state. When excited (which is usually VERY rare), electrons can bump up to higher energy levels like p,d,f,etc. $endgroup$ – khaverim Mar 5 '16 at 3:47. Calcium has 20 electrons and oxygen has 8 electrons, so the TOTAL number of electrons in CaO is 28. If you mean how many valence electrons are used, then calcium uses 2 valence electrons,. Calcium has 20 electrons and m shell is a 3rd energy level. Its electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 instead of 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 1. This is because of Afbau’s principle which states that electrons fill orbitals starting at the lowest available (possible) energy levels before filling higher levels (e.g. 1s before 2s and 4s before 3d).
Calcium can be found in the 4th energy level (row) of the periodic table, and in the 2nd group (column). The first two groups (columns) of the periodic table represent the 's' orbital group. This means that the electron configuration for calcium must end with
The total electron configuration is:

The valence electrons are found in the highest energy level of the electron configuration in the 's' and 'p' orbitals.
In the case of calcium this is
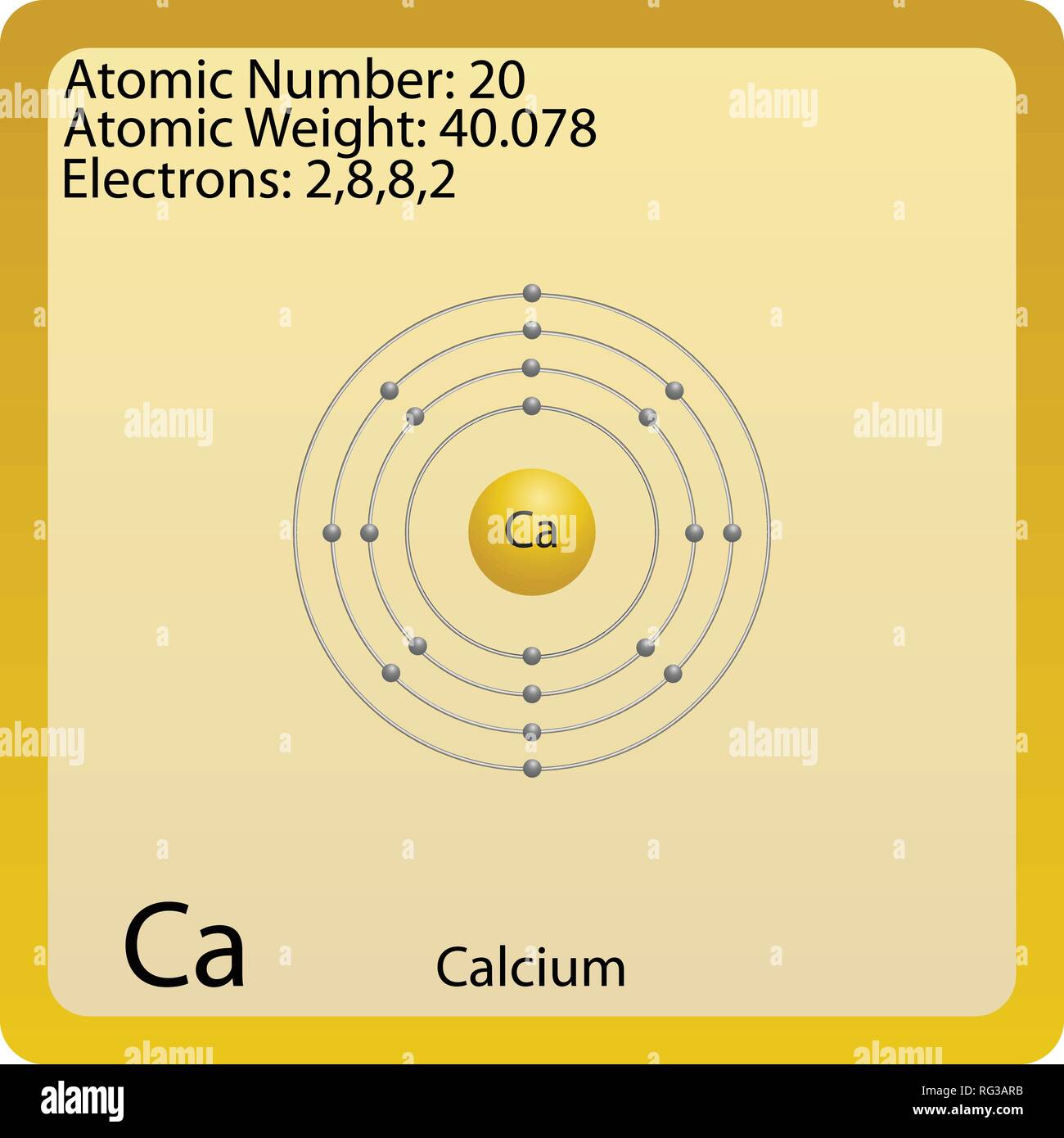
I hope this was helpful.
SMARTERTEACHER
Related questions
June 2018 Chemistry Regents #1-5
Highlight to reveal answers and explanations
Element Ca Protons

Electrons In Calcium 20
Questions | Answer | Links | Explanations |
| 1 Which statement describes the charge and location of an electron in an atom? (1) An electron has a positive charge and is located outside the nucleus. (2) An electron has a positive charge and is located in the nucleus. (3) An electron has a negative charge and is located outside the nucleus. (4) An electron has a negative charge and is located in the nucleus. | 3 | electrons...negative...outside nucleus protons....positive...in nucleus | |
| 2 Which statement explains why a xenon atom is electrically neutral? (1) The atom has fewer neutrons than electrons. (2) The atom has more protons than electrons. (3) The atom has the same number of neutrons and electrons. (4) The atom has the same number of protons and electrons. | 4 | neutral...postive equals negative protons equal electrons | |
| 3 If two atoms are isotopes of the same element, the atoms must have (1) the same number of protons and the same number of neutrons (2) the same number of protons and a different number of neutrons (3) a different number of protons and the same number of neutrons (4) a different number of protons and a different number of neutrons | 2 | isotopes...same number protons different number of neutrons | |
| 4 Which electrons in a calcium atom in the ground state have the greatest effect on the chemical properties of calcium? (1) the two electrons in the first shell (2) the two electrons in the fourth shell (3) the eight electrons in the second shell (4) the eight electrons in the third shell | 2 | valence electrons are the ones that determine chemical properties highest energy level electrons | |
| 5 The weighted average of the atomic masses of the naturally occurring isotopes of an element is the (1) atomic mass of the element (2) atomic number of the element (3) mass number of each isotope (4) formula mass of each isotope | 1 | definition of atomic mass |
